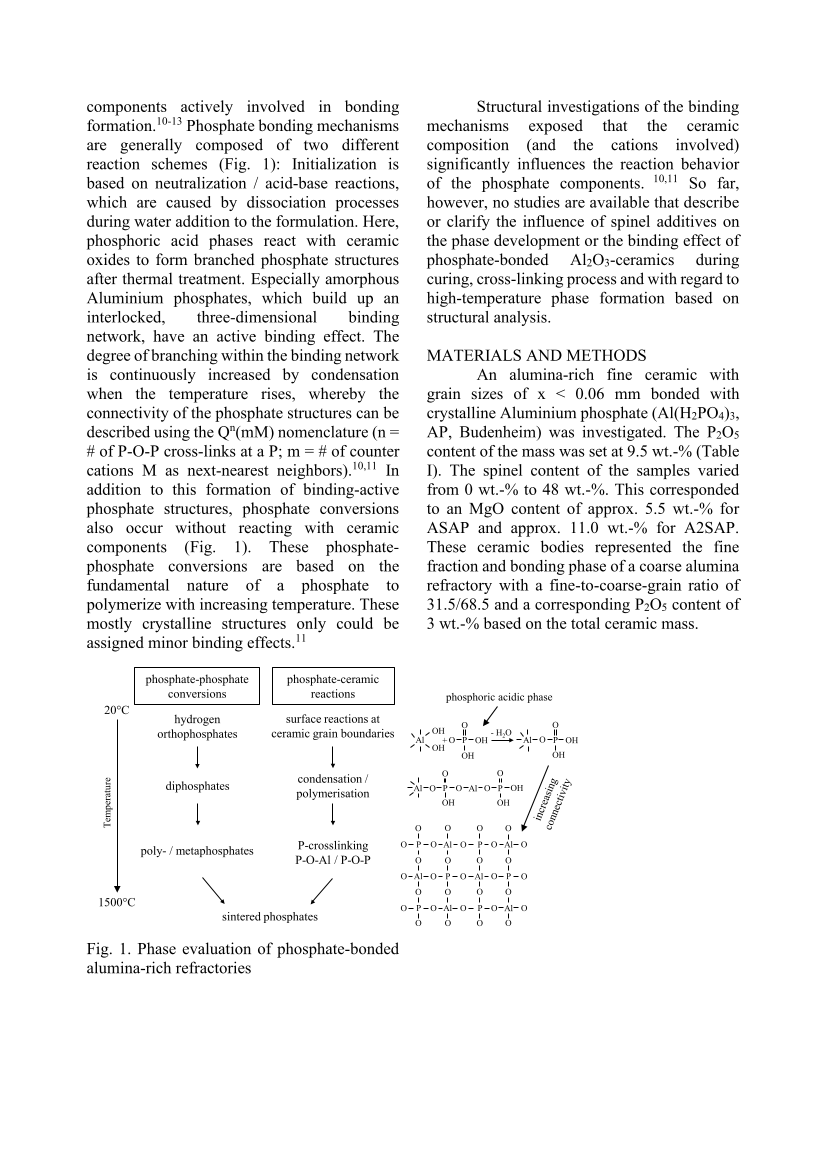
components actively involved in bonding formation.10-13 Phosphate bonding mechanisms are generally composed of two different reaction schemes (Fig. 1): Initialization is based on neutralization / acid-base reactions, which are caused by dissociation processes during water addition to the formulation. Here, phosphoric acid phases react with ceramic oxides to form branched phosphate structures after thermal treatment. Especially amorphous Aluminium phosphates, which build up an interlocked, three-dimensional binding network, have an active binding effect. The degree of branching within the binding network is continuously increased by condensation when the temperature rises, whereby the connectivity of the phosphate structures can be described using the Qn(mM) nomenclature (n = # of P-O-P cross-links at a P m = # of counter cations M as next-nearest neighbors).10,11 In addition to this formation of binding-active phosphate structures, phosphate conversions also occur without reacting with ceramic components (Fig. 1). These phosphate- phosphate conversions are based on the fundamental nature of a phosphate to polymerize with increasing temperature. These mostly crystalline structures only could be assigned minor binding effects.11 Fig. 1. Phase evaluation of phosphate-bonded alumina-rich refractories Structural investigations of the binding mechanisms exposed that the ceramic composition (and the cations involved) significantly influences the reaction behavior of the phosphate components. 10,11 So far, however, no studies are available that describe or clarify the influence of spinel additives on the phase development or the binding effect of phosphate-bonded Al2O3-ceramics during curing, cross-linking process and with regard to high-temperature phase formation based on structural analysis. MATERIALS AND METHODS An alumina-rich fine ceramic with grain sizes of x 0.06 mm bonded with crystalline Aluminium phosphate (Al(H2PO4)3, AP, Budenheim) was investigated. The P2O5 content of the mass was set at 9.5 wt.-% (Table I). The spinel content of the samples varied from 0 wt.-% to 48 wt.-%. This corresponded to an MgO content of approx. 5.5 wt.-% for ASAP and approx. 11.0 wt.-% for A2SAP. These ceramic bodies represented the fine fraction and bonding phase of a coarse alumina refractory with a fine-to-coarse-grain ratio of 31.5/68.5 and a corresponding P2O5 content of 3 wt.-% based on the total ceramic mass. surface reactions at ceramic grain boundaries condensation / polymerisation P-crosslinking P-O-Al / P-O-P sintered phosphates phosphate-phosphate conversions phosphate-ceramic reactions 20°C 1500°C hydrogen orthophosphates diphosphates poly- / metaphosphates Al OH OH + P OH OH O O - H 2 O Al O P OH OH O phosphoric acidic phase Al O P O OH O Al O P OH OH O O Al O P O O O Al O P O O O O O O O P O Al O O P O Al O O O O O P O Al O O P O Al O O O O O Temperature
(c) 2024 American Ceramic Society. All Rights reserved.