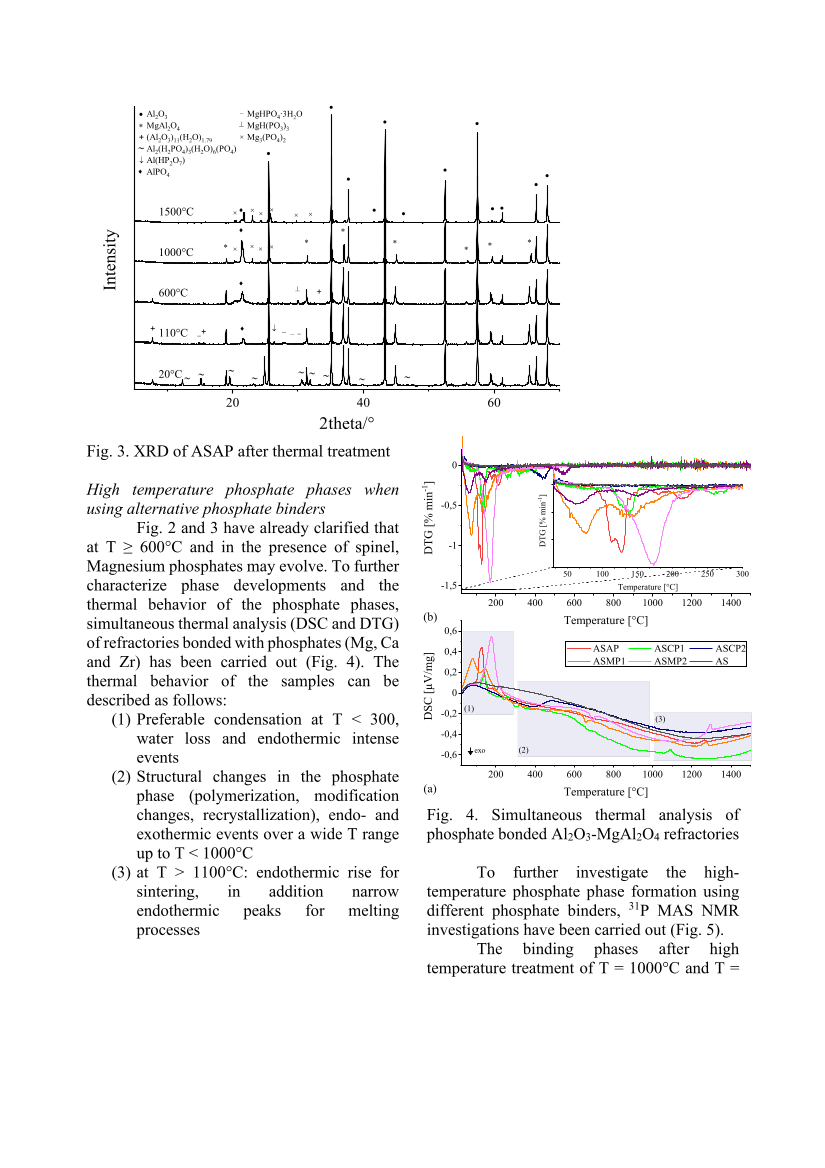
Fig. 3. XRD of ASAP after thermal treatment High temperature phosphate phases when using alternative phosphate binders Fig. 2 and 3 have already clarified that at T ≥ 600°C and in the presence of spinel, Magnesium phosphates may evolve. To further characterize phase developments and the thermal behavior of the phosphate phases, simultaneous thermal analysis (DSC and DTG) of refractories bonded with phosphates (Mg, Ca and Zr) has been carried out (Fig. 4). The thermal behavior of the samples can be described as follows: (1) Preferable condensation at T 300, water loss and endothermic intense events (2) Structural changes in the phosphate phase (polymerization, modification changes, recrystallization), endo- and exothermic events over a wide T range up to T 1000°C (3) at T 1100°C: endothermic rise for sintering, in addition narrow endothermic peaks for melting processes Fig. 4. Simultaneous thermal analysis of phosphate bonded Al2O3-MgAl2O4 refractories To further investigate the high- temperature phosphate phase formation using different phosphate binders, 31 P MAS NMR investigations have been carried out (Fig. 5). The binding phases after high temperature treatment of T = 1000°C and T = 20 40 60 2theta/° • ∗ + Al 2 O 3 MgAl2O4 (Al 2 O 3 ) 11 (H 2 O) 1.79 + + ∗ ∗ • • • • • • • • • • ∗ ∗ ∗ ∗ • 20°C 110°C 600°C 1000°C 1500°C AlPO 4 Al 2 (H 2 PO 4 ) 3 (H 2 O) 6 (PO 4 ) ∼ ∼ ∼ ∼ ∼ ∼ ∼ ∼ ∼ ∼ Al(HP2O7) ♦ ♦ ♦ ♦ ↓ ↓ − MgHPO 4 ∙3H 2 O − ⊥ ⊥ MgH(PO3)3 Mg 3 (PO 4 ) 2 × × × × × × × × × × × • ∗ + − − − ♦ 200 400 600 800 1000 1200 1400 -1,5 -1 -0,5 0 50 100 150 200 250 300 200 400 600 800 1000 1200 1400 -0,6 -0,4 -0,2 0 0,2 0,4 0,6 Temperature [°C] (a) (b) Temperature [°C] Temperature [°C] ASAP ASCP1 ASCP2 ASMP1 ASMP2 AS exo (1) (2) (3) Intensity DTG [% min-1] DTG [% min-1] DSC [µV/mg]
(c) 2024 American Ceramic Society. All Rights reserved.