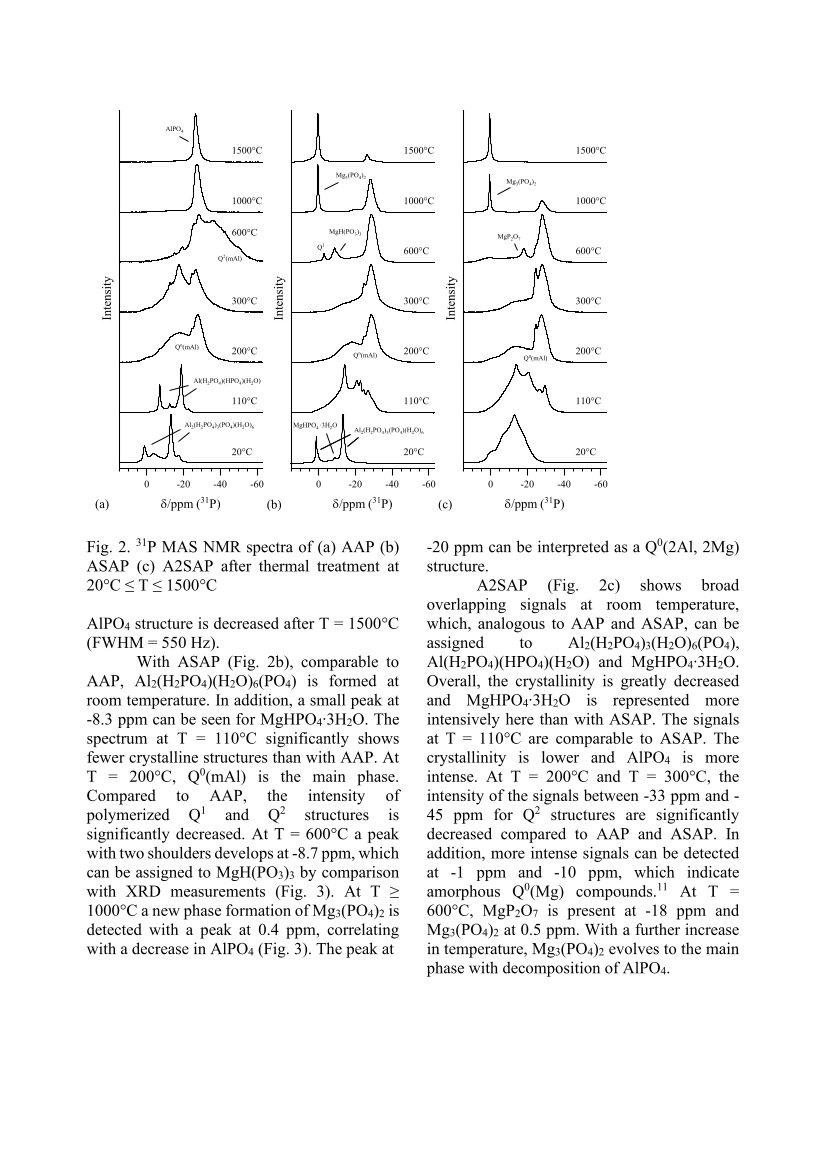
Fig. 2. 31 P MAS NMR spectra of (a) AAP (b) ASAP (c) A2SAP after thermal treatment at 20°C ≤ T ≤ 1500°C AlPO4 structure is decreased after T = 1500°C (FWHM = 550 Hz). With ASAP (Fig. 2b), comparable to AAP, Al2(H2PO4)(H2O)6(PO4) is formed at room temperature. In addition, a small peak at -8.3 ppm can be seen for MgHPO4∙3H2O. The spectrum at T = 110°C significantly shows fewer crystalline structures than with AAP. At T = 200°C, Q0(mAl) is the main phase. Compared to AAP, the intensity of polymerized Q1 and Q2 structures is significantly decreased. At T = 600°C a peak with two shoulders develops at -8.7 ppm, which can be assigned to MgH(PO3)3 by comparison with XRD measurements (Fig. 3). At T ≥ 1000°C a new phase formation of Mg3(PO4)2 is detected with a peak at 0.4 ppm, correlating with a decrease in AlPO4 (Fig. 3). The peak at -20 ppm can be interpreted as a Q0(2Al, 2Mg) structure. A2SAP (Fig. 2c) shows broad overlapping signals at room temperature, which, analogous to AAP and ASAP, can be assigned to Al2(H2PO4)3(H2O)6(PO4), Al(H2PO4)(HPO4)(H2O) and MgHPO4∙3H2O. Overall, the crystallinity is greatly decreased and MgHPO4∙3H2O is represented more intensively here than with ASAP. The signals at T = 110°C are comparable to ASAP. The crystallinity is lower and AlPO4 is more intense. At T = 200°C and T = 300°C, the intensity of the signals between -33 ppm and - 45 ppm for Q2 structures are significantly decreased compared to AAP and ASAP. In addition, more intense signals can be detected at -1 ppm and -10 ppm, which indicate amorphous Q0(Mg) compounds.11 At T = 600°C, MgP2O7 is present at -18 ppm and Mg3(PO4)2 at 0.5 ppm. With a further increase in temperature, Mg3(PO4)2 evolves to the main phase with decomposition of AlPO4. 0 -20 -40 -60 0 -20 -40 -60 0 -20 -40 -60 δ/ppm (31P) 1500°C 1000°C 600°C 300°C 200°C 110°C 20°C 1500°C 1000°C 600°C 300°C 200°C 110°C 20°C 1500°C 1000°C 600°C 300°C 200°C 110°C 20°C (a) (b) (c) Qn(mAl) AlPO 4 Q2(mAl) Al2(H2PO4)3(PO4)(H2O)6 δ/ppm (31P) MgHPO4 ∙3H2O Al(H2PO4)(HPO4)(H2O) Qn(mAl) Q1 MgH(PO3)3 Mg3(PO4)2 δ/ppm (31P) MgP2O7 Mg 3 (PO 4 ) 2 Qn(mAl) Al2(H2PO4)3(PO4)(H2O)6 Intensity Intensity Intensity
(c) 2024 American Ceramic Society. All Rights reserved.