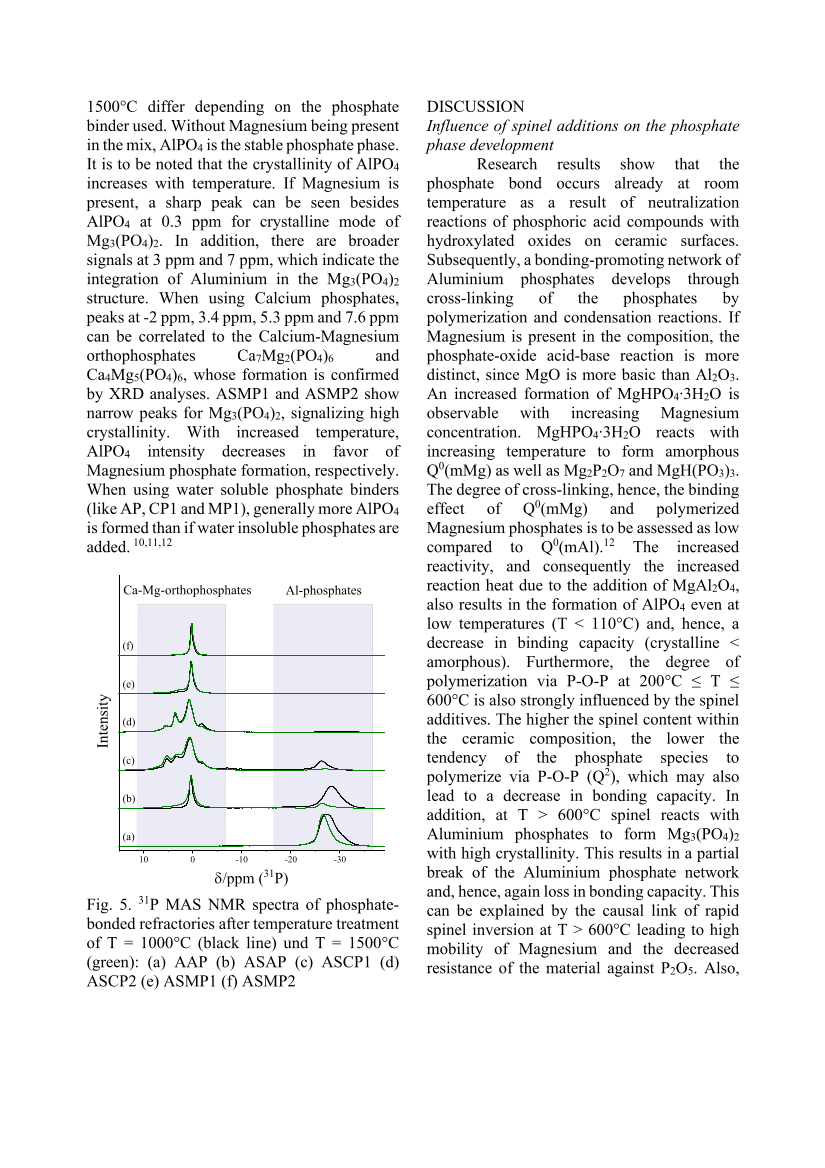
1500°C differ depending on the phosphate binder used. Without Magnesium being present in the mix, AlPO4 is the stable phosphate phase. It is to be noted that the crystallinity of AlPO4 increases with temperature. If Magnesium is present, a sharp peak can be seen besides AlPO4 at 0.3 ppm for crystalline mode of Mg3(PO4)2. In addition, there are broader signals at 3 ppm and 7 ppm, which indicate the integration of Aluminium in the Mg3(PO4)2 structure. When using Calcium phosphates, peaks at -2 ppm, 3.4 ppm, 5.3 ppm and 7.6 ppm can be correlated to the Calcium-Magnesium orthophosphates Ca7Mg2(PO4)6 and Ca4Mg5(PO4)6, whose formation is confirmed by XRD analyses. ASMP1 and ASMP2 show narrow peaks for Mg3(PO4)2, signalizing high crystallinity. With increased temperature, AlPO4 intensity decreases in favor of Magnesium phosphate formation, respectively. When using water soluble phosphate binders (like AP, CP1 and MP1), generally more AlPO4 is formed than if water insoluble phosphates are added. 10,11,12 Fig. 5. 31 P MAS NMR spectra of phosphate- bonded refractories after temperature treatment of T = 1000°C (black line) und T = 1500°C (green): (a) AAP (b) ASAP (c) ASCP1 (d) ASCP2 (e) ASMP1 (f) ASMP2 DISCUSSION Influence of spinel additions on the phosphate phase development Research results show that the phosphate bond occurs already at room temperature as a result of neutralization reactions of phosphoric acid compounds with hydroxylated oxides on ceramic surfaces. Subsequently, a bonding-promoting network of Aluminium phosphates develops through cross-linking of the phosphates by polymerization and condensation reactions. If Magnesium is present in the composition, the phosphate-oxide acid-base reaction is more distinct, since MgO is more basic than Al2O3. An increased formation of MgHPO4∙3H2O is observable with increasing Magnesium concentration. MgHPO4∙3H2O reacts with increasing temperature to form amorphous Q0(mMg) as well as Mg2P2O7 and MgH(PO3)3. The degree of cross-linking, hence, the binding effect of Q0(mMg) and polymerized Magnesium phosphates is to be assessed as low compared to Q0(mAl).12 The increased reactivity, and consequently the increased reaction heat due to the addition of MgAl2O4, also results in the formation of AlPO4 even at low temperatures (T 110°C) and, hence, a decrease in binding capacity (crystalline amorphous). Furthermore, the degree of polymerization via P-O-P at 200°C ≤ T ≤ 600°C is also strongly influenced by the spinel additives. The higher the spinel content within the ceramic composition, the lower the tendency of the phosphate species to polymerize via P-O-P (Q2), which may also lead to a decrease in bonding capacity. In addition, at T 600°C spinel reacts with Aluminium phosphates to form Mg3(PO4)2 with high crystallinity. This results in a partial break of the Aluminium phosphate network and, hence, again loss in bonding capacity. This can be explained by the causal link of rapid spinel inversion at T 600°C leading to high mobility of Magnesium and the decreased resistance of the material against P2O5. Also, 10 0 -10 -20 -30 δ/ppm (31P) Al-phosphates Ca-Mg-orthophosphates (f) (e) (d) (c) (b) (a) Intensity
(c) 2024 American Ceramic Society. All Rights reserved.